A Breakthrough in Early Detection
Discover how DELFI's innovative blood test leverages cutting-edge genomic technology to help detect lung cancer at earliest stages.
DELFI's FirstLook Lung test identifies genome-wide alterations associated with lung cancer at high sensitivity using a simple blood draw.1,2
Intended for individuals deemed eligible for lung cancer screening by the United States Preventative Services Task Force (USPSTF), FirstLook Lung helps determine the likelihood of detecting lung cancer through Low-Dose Computed Tomography (LDCT).
FirstLook Lung is designed to be a convenient first step in detecting lung cancer early, when it is most treatable.
Building Evidence Through Strategic Health System Collaborations
Leading academic medical centers and integrated delivery networks have partnered with DELFI to implement the FirstLook Lung cancer screening solution and, in some instances, contribute to clinical evidence for early detection programs.
These strategically selected health systems - including several of the nation's most respected healthcare institutions - collaborate with our team to optimize lung cancer screening workflows, improve patient outcomes, and demonstrate their commitment to innovative preventive care.
Their experience showcases how adopting cutting-edge technology can transform cancer screening programs while delivering measurable clinical and operational value.
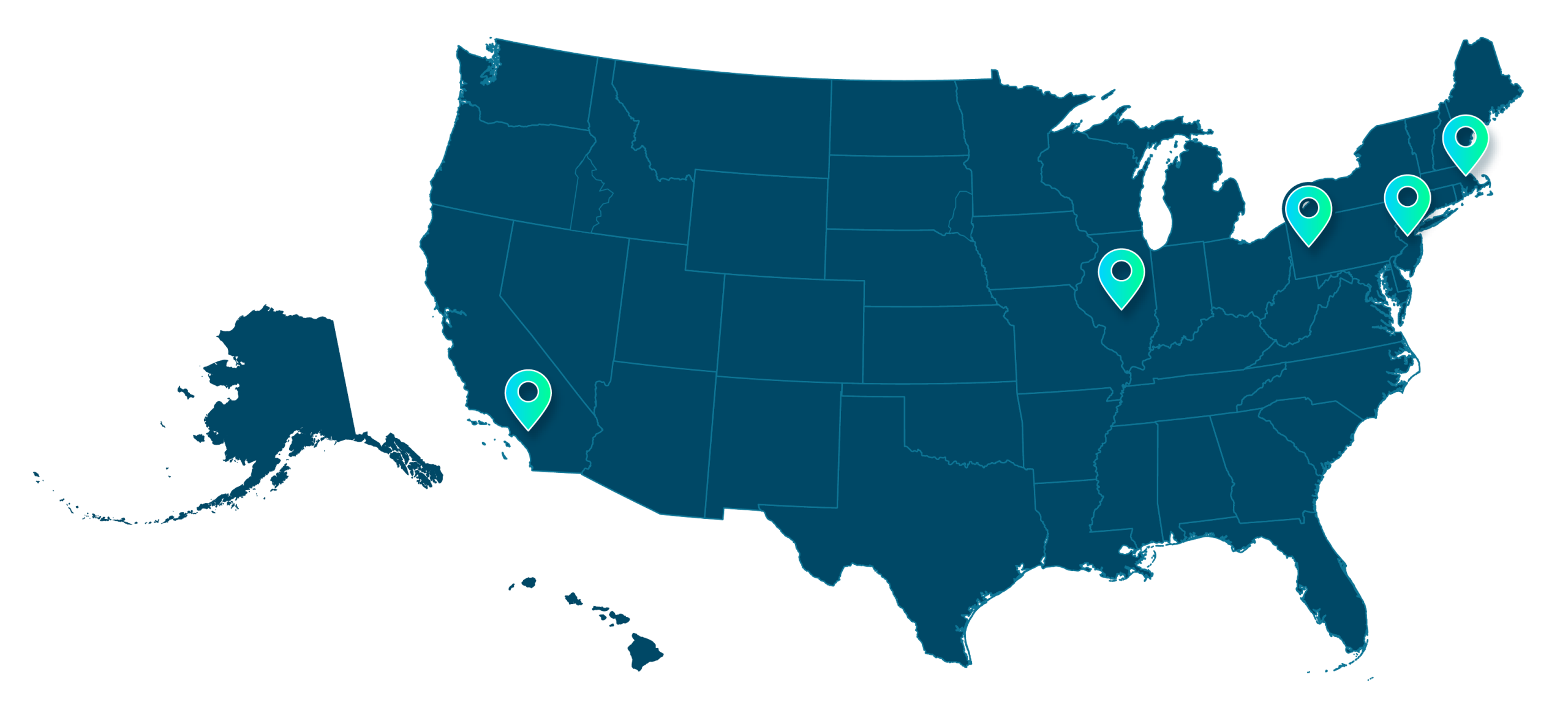
Our health system partners and collaborators include
(alphabetical order):
Read the original validation study1 of the FirstLook Lung test published in Cancer Discovery.
The Advantages of FirstLook Lung Screening
-
99.8% negative predictive value and clinically validated for lung cancer2
-
Earlier detection enables earlier intervention
-
Treating cancer sooner focuses resources on ensuring better outcomes
-
Improved patient engagement in early lung cancer detection
-

Seamless integration into existing healthcare systems with best-practice alerts and advanced EHR integration capabilities
-
A simple lab test-ordering process; no extensive training or additional equipment is needed
-
Patient and provider-focused educational materials to support outreach teams
Important Insights: Just a Click Away
Gain a deeper understanding of our fragmentomics technology by downloading and reading our brochure. This document provides an overview of DELFI and how we utilized our technology to create the FirstLook Lung cancer screening test.

Ready to Enhance Lung Cancer Screening in Your Network?
Schedule a meeting with our partnership executives to discuss how our innovative genomics solution with FirstLook can become part of your lung cancer screening program. Learn about the applications of fragmentomics research and non-invasive cancer testing. Let us help you take the next steps in improving your organization's patient care and resource allocation through next-generation blood-based tests and cfDNA testing.
References: 1. Mazzone, P.J., Bach, P.B., Carey, J., et al. Cancer Discovery. 10.1158 – 2159-8290. CD-24-0519. (2024). 2. Unpublished data on file.
FirstLook Lung is intended to be an adjunct, qualitative aid evaluating adults eligible for LDCT lung cancer screening. It is designed to supplement, not supplant, recommended screening tests for lung cancer in the population described in the Indications for Use, including LDCT examination.
The FirstLook Lung test is a laboratory-developed test. It was developed and its performance characteristics determined by DELFI Diagnostics. It has not been cleared or approved by the FDA. The laboratory is regulated under the Clinical Laboratory Improvement Act (CLIA) as qualified to perform high complexity clinical tests. This test is used for clinical purposes. It should not be regarded as investigational or for research.
FirstLook is a trademark of DELFI Diagnostics, Inc. used worldwide.