Get a FirstLook at lung cancer
Lung cancer can be found early, but 84% of eligible adults are not getting the nationally recommended LDCT screening.1

Overcome lung cancer screening barriers with an easy first step.
Introducing FirstLook, a simple blood test designed to be the first step in detecting lung cancer early, when it’s most treatable.
*The United States Preventive Services Task Force (USPSTF) recommends annual low-dose CT screening for lung cancer in adults aged 50-80 years who have a ≥20 pack-year smoking history, who currently smoke, or who have quit within the past 15 years.2
The new FirstLook blood test offers a convenient, accessible approach to enhance lung cancer screening. FirstLook helps determine the likelihood of detecting lung cancer through low-dose CT (LDCT).
Addressing Your Screening Challenge
Lung cancer screening faces unique barriers – patient anxiety about radiation and scheduling difficulties for LDCT. FirstLook addresses these barriers by offering the first step as a simple blood draw during routine visits.
FirstLook Lung is a next-generation sequencing laboratory-developed test (LDT) of plasma-cell-free DNA that analyzes DNA fragment sizes in blood to indicate the possible presence of lung cancer.
Seamless Practice Integration
Like other routine blood draws, FirstLook can be completed in your office or by your institution’s phlebotomy lab. No special equipment, no patient prep, and results delivered in 10-14 days.
Don't have time today to read?
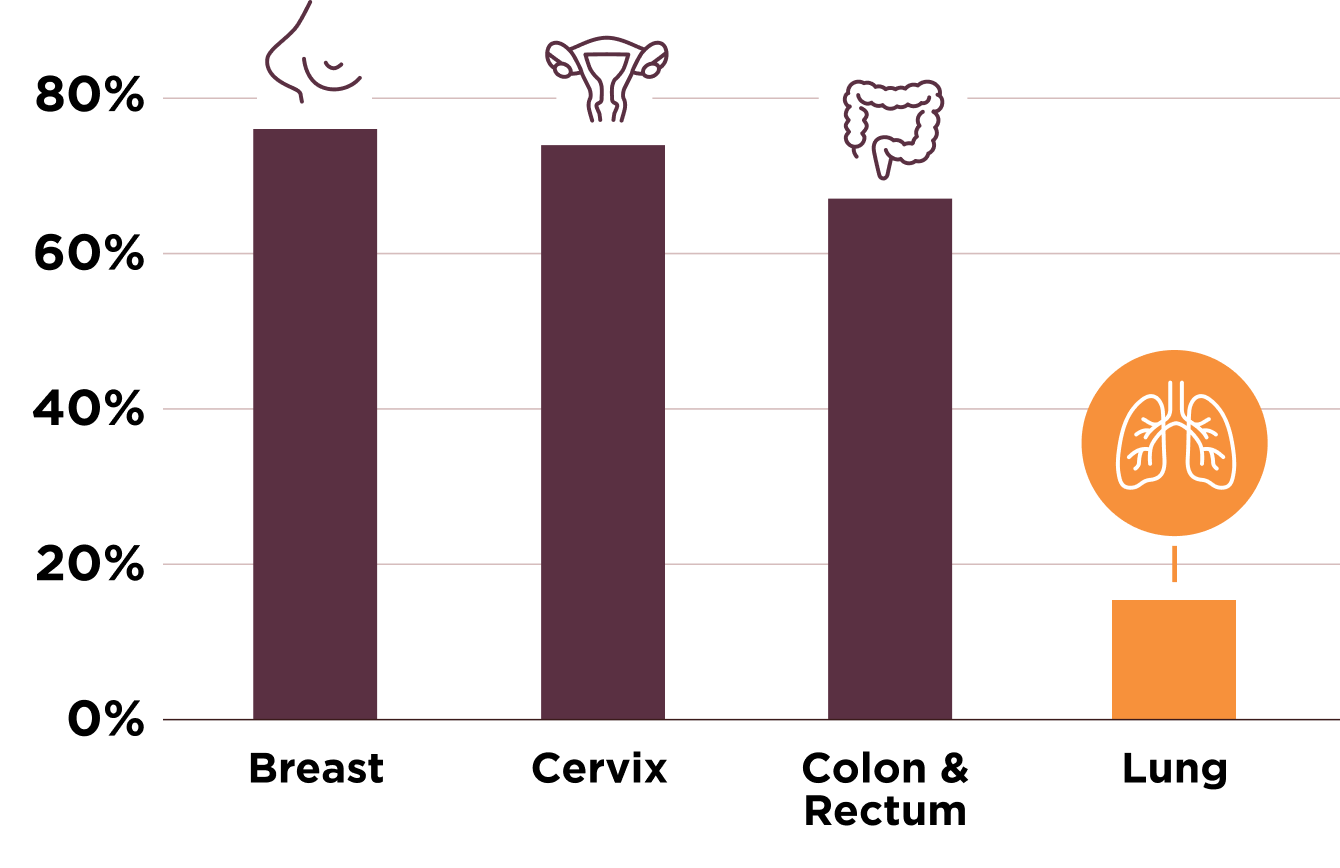
Lung cancer screening has fallen behind other cancer screenings.
Screening Rates: Eligible US Adults1,5
Detecting lung cancer early saves lives.7,8
Invented with a team of physicians, FirstLook is designed to be the first step in detecting lung cancer early.

Understand your patient's lung health at a glance.
FirstLook provides binary results:

An Elevated result
means a higher likelihood of lung cancer detected by low-dose CT (LDCT).

A Not Elevated result
suggests a lower likelihood that LDCT will detect lung cancer.
Clinically impactful performance
FirstLook was validated in a prospective, case-controlled, observational study.
99.8% NPV
In individuals with a Not Elevated result, the chance of detecting lung cancer by LDCT is 2 in 1000.9
79 NNS
About 79 people with an Elevated result need an LDCT to find one person with lung cancer compared with an NNS of 143 for anyone screen eligible.9
5.2X RR
The likelihood of a lung cancer being found by LDCT for individuals with an Elevated result is 5.2 times as high as for those with a Not Elevated result.9
NNS: Number needed to screen, NPV: Negative predictive value, RR: Relative risk.
FirstLook supports lung cancer screening decisions across age groups
The blood test provides additional risk stratification information to help guide LDCT screening decisions.
Clinical Integration
An elevated result indicates a higher likelihood that LDCT will be productive and is the next best step for the patient, while a not elevated result can help inform shared decision-making about screening timing.

It's time to take a FirstLook at lung cancer. Get your patients tested today.
Provider Support You Can Count On
- Results interpretation support and clinical consultation available
- Patient education and counseling materials provided
- Live Client Services support 5 days a week, 8am-5pm ET
FirstLook Lung integrates with your existing workflow as part of routine appointments.
Workflow Steps:
Blood draw
Routine blood collection
Return of results
Simple binary results delivered
Results support
To facilitate informed decisions and follow-up
Provider Support You Can Count On
FirstLook is a laboratory-developed test. Our team works with you and your patients to verify benefits and explore coverage options. Contact Client Services for coverage questions.
Contact Information
Contact DELFI Diagnostics Client Services for questions about accessing the Provider Portal and ordering FirstLook Lung.
We look forward to meeting you!
Learn more about FirstLook Lung and DELFI’s fragmentomics approach at upcoming industry meetings:
-

Visit the DELFI exhibit at the AACR 2026 conference in San Diego, CA, April 17-22, to learn about our latest cancer research on screening, monitoring, and technology advancements.
-

Meet DELFI representatives at the ATS 2026 conference in Orlando, FL, May 17-20, to hear exciting updates about our Firstlook Lung single-tumor, blood-based test for lung cancer screening in USPSTF-eligible patients.
Indications for Use
FirstLook is indicated as an adjunct assessment tool for individuals deemed as high risk by the USPSTF and eligible for lung cancer screening (50-80 years of age, 20 pack-years of smoking, currently smoking or has quit within 15 years).
Laboratory Information
The FirstLook Lung test is a laboratory-developed test. This test was developed, and its performance characteristics were determined by DELFI Diagnostics. It has not been cleared or approved by the US Food and Drug Administration (FDA). The laboratory is regulated under the Clinical Laboratory Improvement Act (CLIA) as qualified to perform high-complexity clinical tests. The test is used for clinical purposes. It should not be regarded as investigational or for research.
References: 1. State of Lung Cancer | Key Findings | American Lung Association. Accessed December 21, 2024. 2. Wang GX, Baggett TP, Pandharipande PV, et al. Barriers to lung cancer screening engagement from the patient and provider perspective. Radiology. 2019;290(2):278-287. doi:10.1148/radiol.2018180212. 3. Meza R, Jeon J, Toumazis I, et al. Evaluation of the benefits and harms of lung cancer screening with low-dose computed tomography: modeling study for the US Preventive Services Task Force. JAMA. 2021;325(10):988-997. doi:10.1001/jama.2021.1077 4. NIH National Cancer Institute. CancerStat Facts: Common Cancer Sites. 2023. 5. American Cancer Society. Cancer Prevention & Early Detection Facts & Figures 2023-2024. 6. Cancer-Healthy People 2030 | health.gov. https://health.gov/healthypeople/objectives-and-data/browse-objectives/cancer. 7. National Lung Screening Trial Research Team; Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):395-409. doi:10.1056/NEJMoa1102873 8. de Koning HJ, Meza R, Plevritis SK, et al. Benefits and harms of computed tomography lung cancer screening strategies: a comparative modeling study for the U.S. Preventive Services Task Force. Ann Intern Med. 2014;160(5):311-320. doi:10.7326/M13-2316 9. Unpublished data on file. 10. Bach PB, Kattan MW, Thornquist MD, et al. Variations in lung cancer risk among smokers. J Natl Cancer Inst. 2003;95(6):470-478.