represents a paradigm shift in monitoring treatment efficacy
DELFI-TF: Advanced Cancer Monitoring When It Matters Most
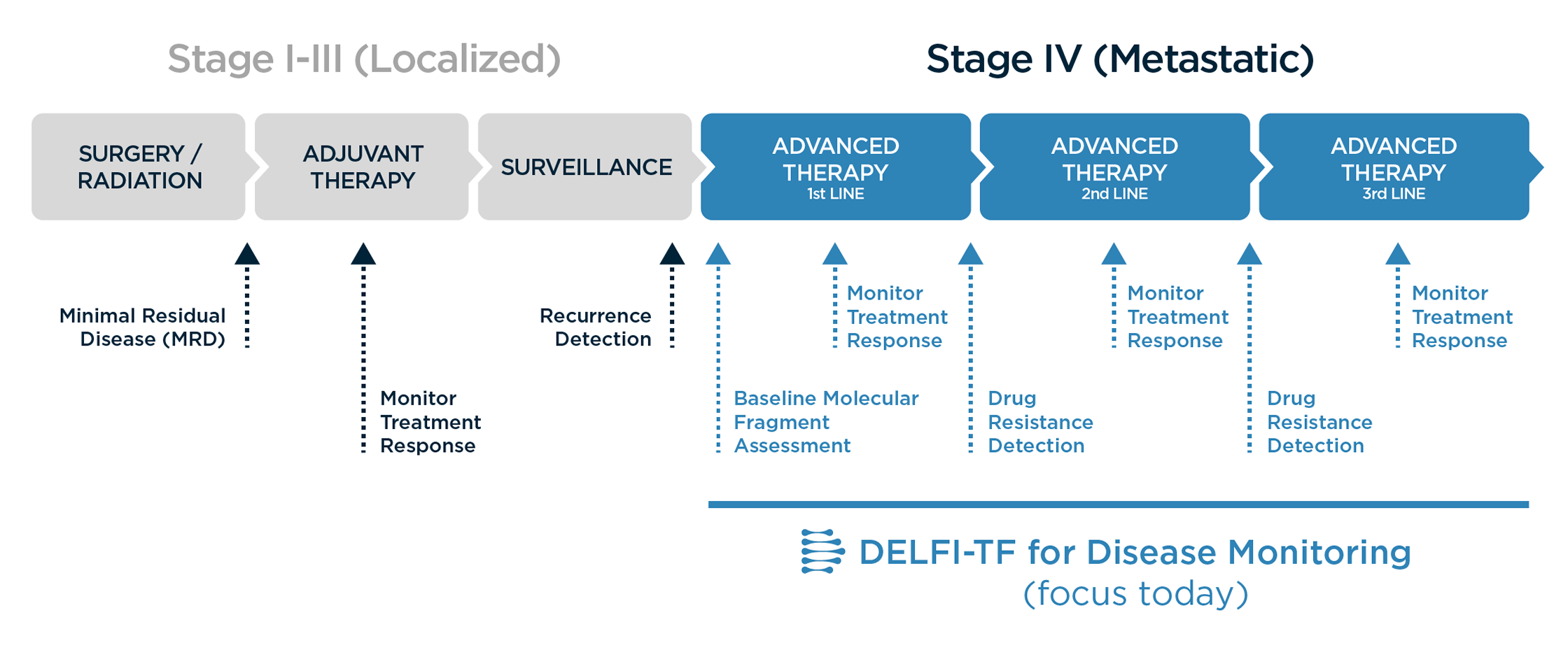
DELFI-TF provides critical insights throughout the metastatic cancer journey. Our technology excels in Stage IV settings across all treatment lines, delivering:
-
Baseline Assessment
Establish molecular profiles before treatment begins -
Response Monitoring
Track therapeutic effectiveness during each line of therapy -
Resistance Detection
Identify emerging resistance patterns earlier than traditional methods -
Continuous Insight
Maintain visibility across the entire treatment journey
With less than 1 mL of plasma, our low-cost solution eliminates the barriers of traditional monitoring methods, giving your development team earlier signals and clearer direction when therapeutic decisions are most critical.
DELFI-TF Industry Adoption:
Powering Development Decisions Today
Since its introduction in February 2024, DELFI-TF has been integrated into the clinical development programs of 25% of the top 20 pharmaceutical companies.
DELFI-TF is actively guiding critical oncology drug development decisions. Our breakthrough technology is validated through rigorous peer-reviewed research published in prestigious scientific journals including Nature and Cancer Discovery, establishing a solid foundation of evidence supporting our approach.
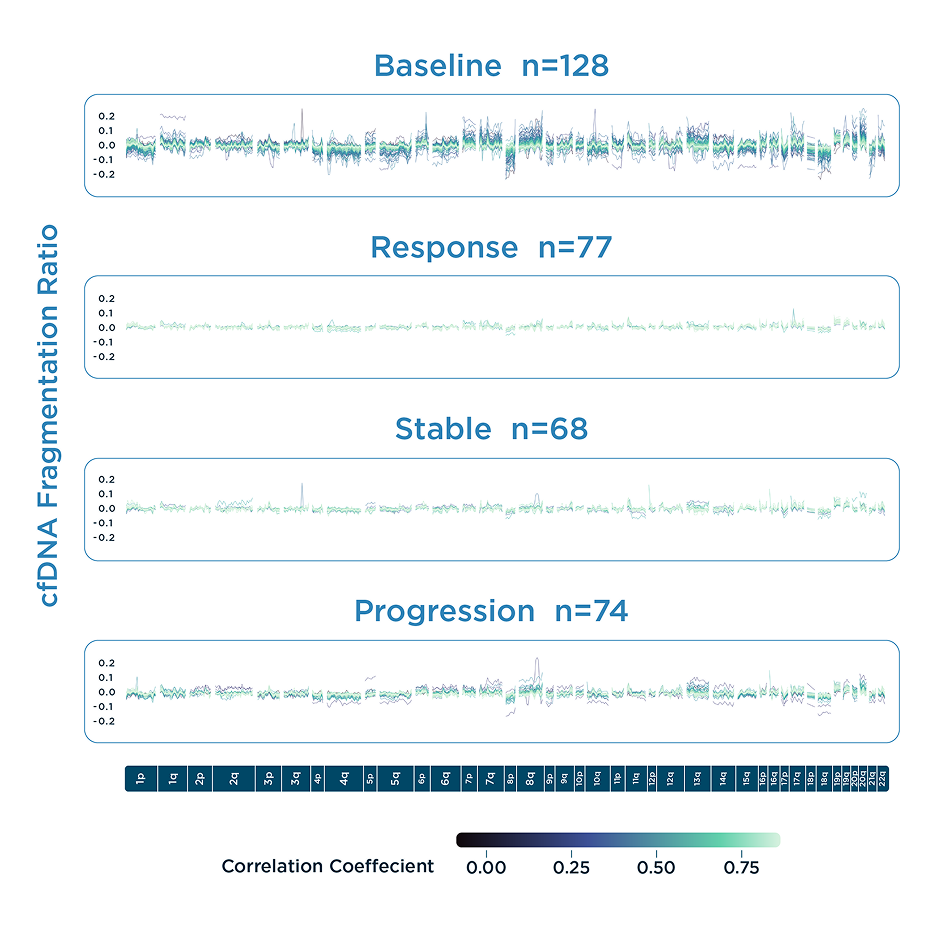
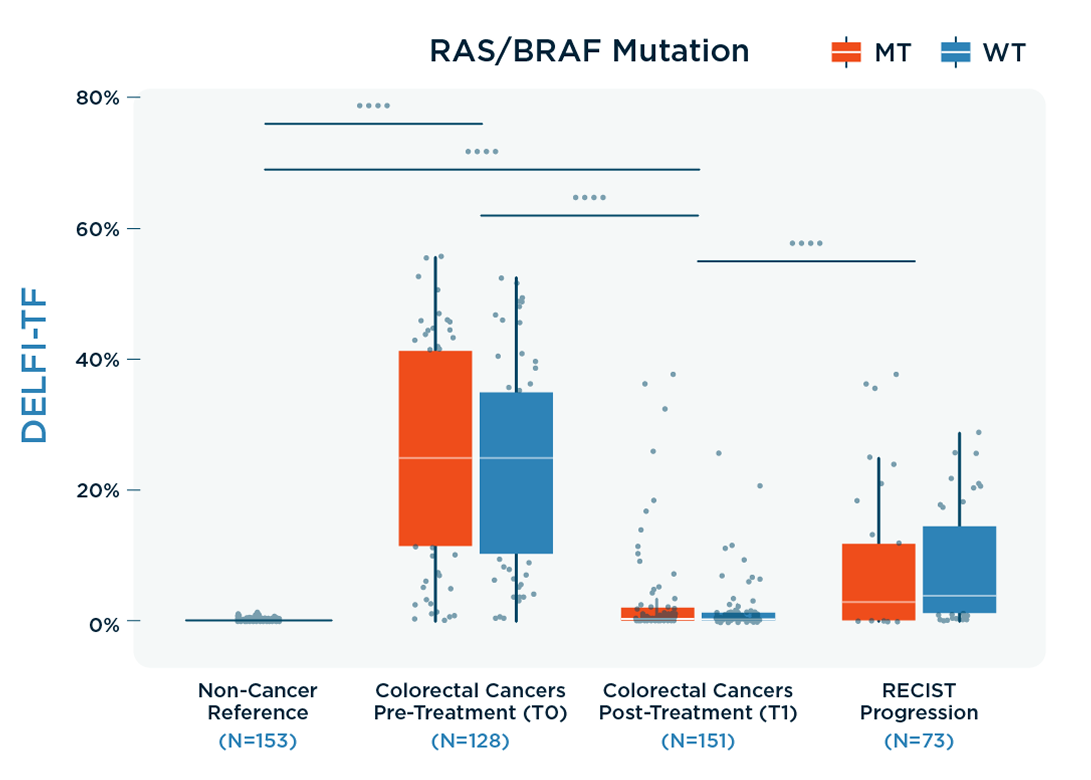
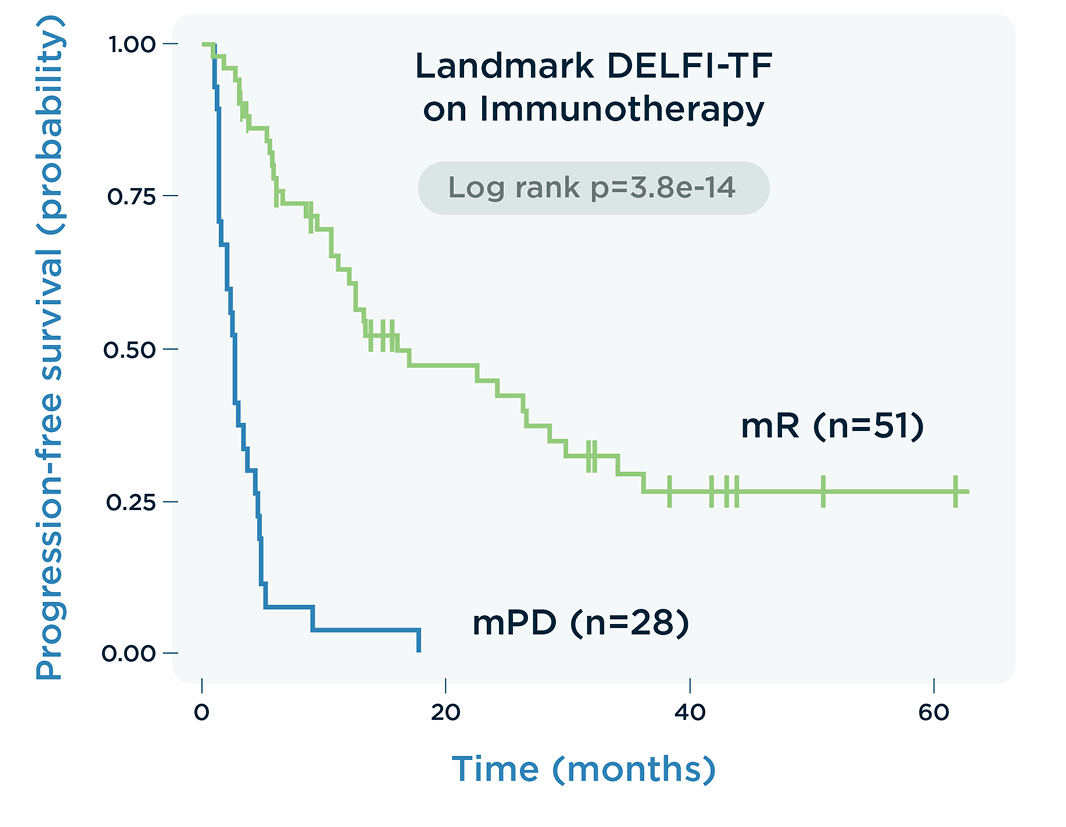
DELFI-TF has equivalent performance to traditional MAF analysis in predicting therapy response, indicating progressive disease earlier over RECIST imaging.
Our software-driven platform approach, backed by access to over 150,000 screening and monitoring samples, ensures continuous improvement through machine learning while maintaining cost-effectiveness.
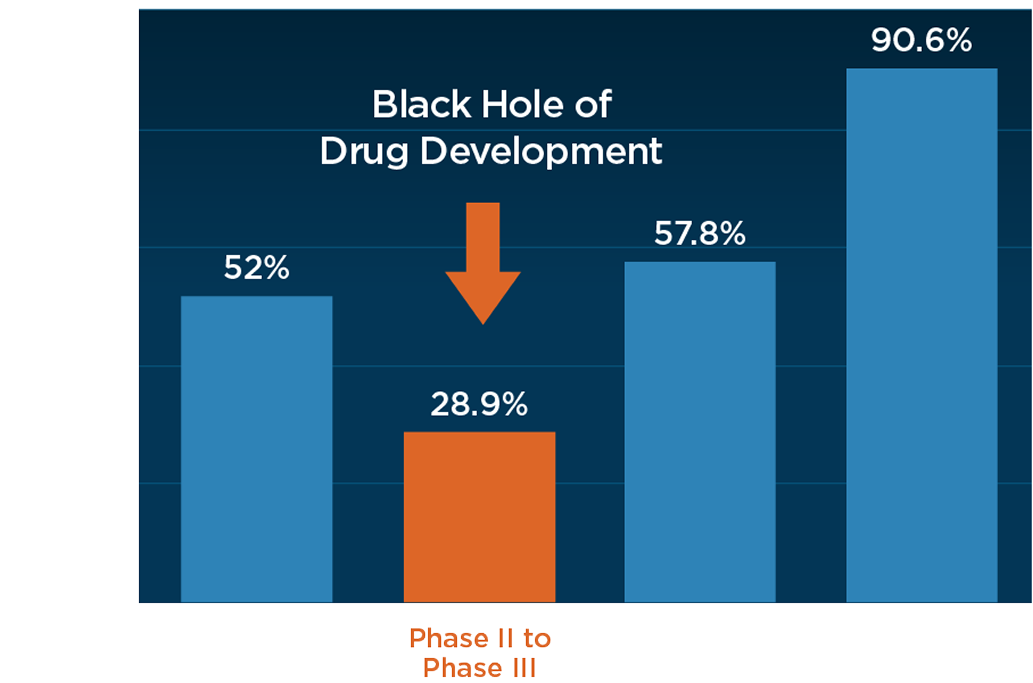
The Critical Efficacy Gateway
The challenging transition between Phase II and Phase III represents oncology’s most significant development hurdle. Over 70% of promising compounds in Phase II trials fail to demonstrate sufficient efficacy to advance into Phase III. Most failures are due to a lack of efficacy (effectiveness) rather than safety or toxicity concerns.
Early Detection of Molecular Response
Industry leaders implement rigorous molecular response monitoring through mutation/MAF assays and precision imaging during Phase Ia/Ib trials to improve Phase II outcomes. This strategic approach delivers valuable efficacy insights but requires substantial resource allocation, specialized expertise, and significant financial investment.
High cost
Tissue and high volumes of plasma required
High failure rate
Long turnaround
Imaging challenges
pseudoprogression, information is not always available or practical, and measurement variability
Reimagining Response Monitoring: Simple, Affordable, Actionable
What if comprehensive ctDNA monitoring was accessible for every patient, drug, and treatment cycle?
What if a streamlined plasma-only assay could deliver precise tumor fraction data for early clinical trials in as few as 10 business days?
What if more data, less complexity, and lower costs could drive your development decisions?

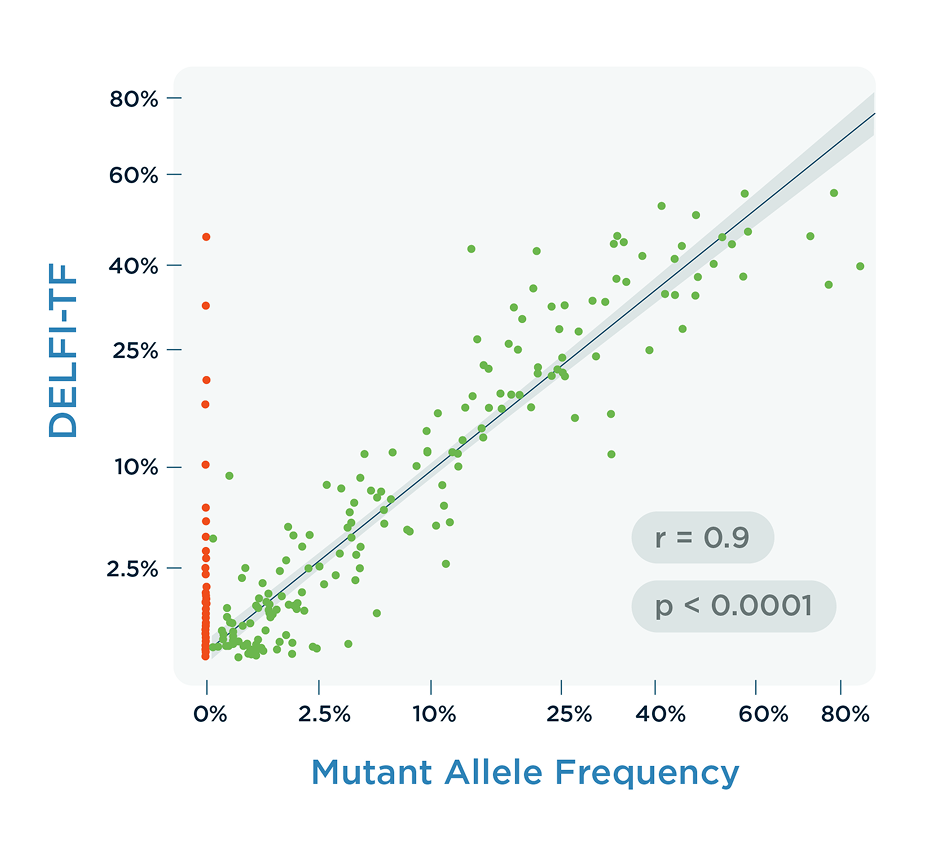
DELFI-TF delivers a genome-wide measure of the proportion of cfDNA derived from a tumor. It is highly correlated with the Mutant Allele Frequency (MAF) that is often used to evaluate treatment response and resistance to immunotherapies in advanced cancer patients. DELFI-TF requires very little plasma, has a low cost of processing, and is not confounded by clonal hematopoiesis or driver mutation switches.

Co-Founder, Senior Scientific Advisor
Tumor-Agnostic Monitoring: The DELFI Advantage
- While cell-free DNA offers tremendous potential for cancer diagnostics, traditional approaches are constrained by the need for tumor-specific mutation data.
- DELFI overcomes this fundamental limitation through our pioneering genome-wide cfDNA fragmentation analysis.
- DELFI-TF scores strongly correlate with circulating tumor DNA (ctDNA) levels, as measured by Mutant Allele Frequency (MAF), even in cases where mutations are undetectable.
- This proprietary technology delivers a tumor and mutation-independent monitoring solution, enabling dynamic treatment response assessment across diverse cancer types and therapeutic interventions.
- Requiring just 800µl of plasma, DELFI-TF delivers comprehensive treatment response monitoring across all tumor types and therapies.
- It typically produces results in 10-14 business days and has a 99% success rate, but most partners receive actionable results sooner.*
- DELFI-TF accurately predicts tumor burden in patients with different types of solid cancer (colorectal, lung, pancreas, breast, melanoma, and head and neck cancers).
Clinical Validation:
Quantifying Tumor Burden Without Mutation Profiling
- DELFI-TF’s groundbreaking technology enables precise tumor burden quantification using only a small plasma sample.
- Our validated approach analyzes genome-wide cfDNA fragmentation patterns, eliminating the need for tumor tissue and prior mutation identification.
- This mutation- and tumor-agnostic methodology delivers accurate response monitoring across diverse cancer types and treatment modalities, transforming how developers assess therapeutic efficacy.
- This was developed and validated using data from individuals with and without identified mutations (as shown in “RAS/BRAF Mutation” DELFI-TF figure).
Technical Superiority:
Redefining Monitoring Performance
Sample requirements:
Only 800µl plasma needed
Turnaround time:
Results in as few as 10 business days*
Success rate:
~99% completion rate
Multi-cancer application:
Validated across multiple tumor types
*Turnaround time depends on study size. Typically results are returned within 10-14 business days (for each step within a Phase Ia dose escalation trial) or 14-30 business days (for larger studies of banked samples).
Get the Full Technical Picture
Take the next step toward optimized response monitoring. Request our technical specification sheet today to review complete performance data, implementation requirements, and compatibility specifications for your development program.
Take Action Today
Schedule an appointment with our Research Services team to discuss how DELFI-TF can accelerate your drug development decisions and provide earlier efficacy signals for your oncology pipeline.
Explore the Validated Science
Download our peer-reviewed validation studies.